
Different sequences of events can lead to different hazardous situations and risks, each contributing to the overall residual risk.

The ISO 24971 provides different inputs that can be used as starting point for the evaluation of the residual risks, for examples: The manufacturer is responsible for determining an appropriate method. There is no preferred way for evaluating the overall residual risk. It is not possible to perform overall residual risks evaluation just by adding up all the single residual risks. It consists in an overall evaluation of the residual risks, meaning the risks after the implementation of risks control measures. The evaluation of the overall residual risks is an important moment of the risk management process.

When the probability of occurrence of harm cannot be estimated, it is necessary to evaluate the risk on the basis of the severity of harm alone.
#Matrix iso 14971 2012 free download software#
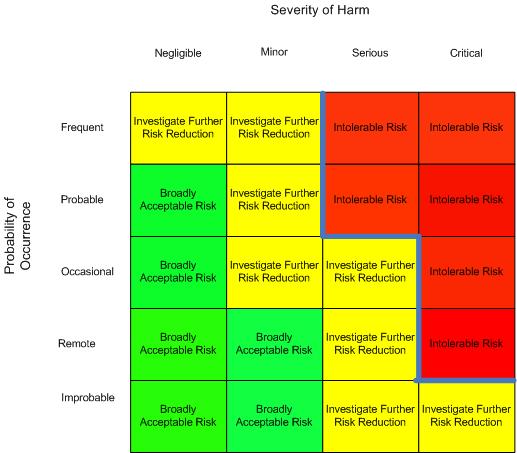
Typical examples are software failures or misuse situations. Otherwise, a qualitative method based is preferable over a quantitative estimate with a high level of uncertainty.Ī particular attention shall be posed to the situation where the probability of occurrence cannot be estimated. When sufficient data are available to estimate the probability of occurrence of harm with adequate confidence, a quantitative method should be used. The risk estimation can be performed by evaluating:įor the estimation of the probability of occurrence, two different methods can be used: quantitative and qualitative. The Process of Risk Estimation According to ISO/TR 24971Ī discussion on the process of risk analysis and risk estimation has already been performed within QualityMedDev blog.Įach identified risks need to be estimated in terms of providing a ranking on the specific risks. In this article we will have a deep dive in the ISO 24971, highlighting the most important requirements and the associated methodologies for the implementation of an efficient risk management process, compliant with the most significant quality management system regulations ( ISO 13485, 21 CFR 820) and other applicable regulatory requirements (EU MDR 2017/745).
#Matrix iso 14971 2012 free download update#
A first revolution of the risk management process carried in 2019 with the publication of the updated version of ISO 14971, which now have been further supported by the update of the related technical report ISO 24971. With the publication of new European Medical Device Regulation 2017/745, the interconnection of the risk management processes with other fundamental processes of the medical device world (clinical evaluation, post-market surveillance, design and development) became more and more evident. The ISO 24971 can be considered a guideline in the implementation of ISO 14971:2019. It is impossible to deny the importance of risk management process for medical device organization and the recent publication of the updated version of ISO 24971 is a valuable tool for the use of risk management process to improve quality, safety and efficacy of the medical devices in the field.


 0 kommentar(er)
0 kommentar(er)
